Welcome to the fascinating world of acids, bases, and salts! This acids bases and salts worksheet is your passport to unlocking the secrets of these fundamental chemical concepts. Prepare to dive into a realm where acidity and basicity dance, and salts emerge as the products of their interactions.
Throughout this guide, we’ll explore the definitions, properties, and reactions of acids, bases, and salts. We’ll also venture into their practical applications, revealing their significance in everyday life and various scientific fields.
Acids and Bases Concepts
Acids and bases are two fundamental chemical concepts that play a vital role in various chemical reactions and everyday life. Understanding their properties and behavior is essential in chemistry.
The concept of acids and bases has evolved over time, leading to different theories that define their characteristics. Three prominent theories are the Arrhenius theory, Bronsted-Lowry theory, and Lewis theory.
Arrhenius Theory
The Arrhenius theory, proposed by Svante Arrhenius in the late 19th century, defines acids as substances that produce hydrogen ions (H+) when dissolved in water, while bases are substances that produce hydroxide ions (OH-) when dissolved in water.
According to this theory, a strong acid is a substance that completely dissociates in water, releasing all its hydrogen ions. Examples of strong acids include hydrochloric acid (HCl) and sulfuric acid (H2SO4). A weak acid, on the other hand, only partially dissociates in water, releasing only a small fraction of its hydrogen ions.
Examples of weak acids include acetic acid (CH3COOH) and carbonic acid (H2CO3).
Similarly, a strong base is a substance that completely dissociates in water, releasing all its hydroxide ions. Examples of strong bases include sodium hydroxide (NaOH) and potassium hydroxide (KOH). A weak base, on the other hand, only partially dissociates in water, releasing only a small fraction of its hydroxide ions.
Examples of weak bases include ammonia (NH3) and pyridine (C5H5N).
Bronsted-Lowry Theory
The Bronsted-Lowry theory, proposed by Johannes Bronsted and Thomas Lowry in the early 20th century, defines acids as substances that can donate protons (H+), while bases are substances that can accept protons.
According to this theory, an acid-base reaction involves the transfer of a proton from an acid to a base. The acid is called the proton donor, while the base is called the proton acceptor.
The Bronsted-Lowry theory is more general than the Arrhenius theory because it does not restrict acids and bases to aqueous solutions. It can also be applied to non-aqueous solvents, such as liquid ammonia or acetic acid.
Lewis Theory
The Lewis theory, proposed by Gilbert N. Lewis in the early 20th century, defines acids as substances that can accept an electron pair, while bases are substances that can donate an electron pair.
According to this theory, an acid-base reaction involves the formation of a covalent bond between the acid and the base. The acid is called the electron-pair acceptor, while the base is called the electron-pair donor.
The Lewis theory is the most general of the three theories because it can be applied to a wide range of chemical reactions, including those that do not involve the transfer of protons.
pH and its Significance
pH is a measure of the acidity or alkalinity of a solution. It is defined as the negative logarithm of the hydrogen ion concentration in a solution.
The pH scale ranges from 0 to 14, with 7 being neutral. Solutions with a pH below 7 are acidic, while solutions with a pH above 7 are basic.
pH is a critical factor in many chemical reactions and biological processes. For example, the pH of blood is tightly regulated to maintain a narrow range necessary for optimal functioning.
The acids, bases, and salts worksheet is a great resource for learning about these important chemical concepts. If you’re looking for more practice, check out the nys landscapes lab answer key . This resource provides step-by-step solutions to the worksheet problems, so you can check your work and make sure you’re understanding the material.
Once you’ve mastered the basics of acids, bases, and salts, you’ll be well on your way to understanding more complex chemical reactions.
Acid-Base Reactions: Acids Bases And Salts Worksheet
Acid-base reactions are chemical reactions that involve the transfer of protons (H+ ions) between reactants. These reactions play a crucial role in various chemical processes, including neutralization, hydrolysis, and salt formation.
Neutralization Reactions
Neutralization reactions occur when an acid and a base react in stoichiometric amounts, resulting in the formation of a salt and water. The products of a neutralization reaction are typically neutral, meaning they have a pH of 7.
Hydrolysis Reactions
Hydrolysis reactions involve the reaction of an acid or a base with water. In the case of an acid, hydrolysis produces hydronium ions (H3O+) and the conjugate base of the acid. Conversely, hydrolysis of a base produces hydroxide ions (OH-) and the conjugate acid of the base.
Salt Formation Reactions
Salt formation reactions occur when a strong acid reacts with a strong base, or when a weak acid reacts with a weak base. These reactions result in the formation of a salt, which is an ionic compound composed of positively charged cations and negatively charged anions.
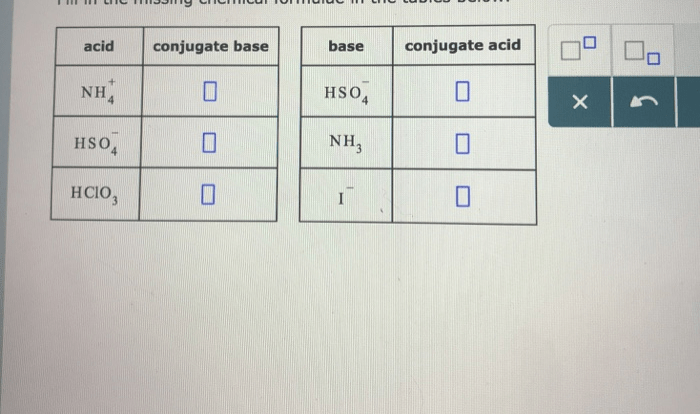
Conjugate Acid-Base Pairs
In acid-base reactions, the conjugate acid-base pair concept is essential. A conjugate acid is the species formed when a base accepts a proton, while a conjugate base is the species formed when an acid donates a proton. The conjugate acid-base pair is related by the following equilibrium:“`Acid + Base ⇌ Conjugate Base + Conjugate Acid“`For example, in the neutralization reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH), the conjugate acid-base pair is H3O+ (hydronium ion) and Cl- (chloride ion).
Salt Formation and Properties
Salts are ionic compounds that form when an acid and a base react. They are composed of positively charged ions (cations) and negatively charged ions (anions).
Formation of Salts
When an acid and a base react, they undergo a neutralization reaction, which produces a salt and water. The reaction can be represented as follows:
Acid + Base → Salt + Water
For example, when hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH), it forms sodium chloride (NaCl) and water:
HCl + NaOH → NaCl + H2O
Properties of Salts
Salts have several characteristic properties, including:
- Solubility:Most salts are soluble in water. When they dissolve, they dissociate into their constituent ions.
- Conductivity:Salts conduct electricity when dissolved in water. This is because the ions in the solution can move freely and carry an electric current.
- Reactivity:Salts can react with other substances to form new compounds. For example, sodium chloride can react with silver nitrate to form silver chloride and sodium nitrate.
Types of Salts
There are many different types of salts, each with its own unique properties. Some common types of salts include:
- Sodium chloride (NaCl):Table salt is the most common type of salt. It is used as a seasoning and a preservative.
- Potassium chloride (KCl):Potassium chloride is used as a fertilizer and a substitute for table salt for people with high blood pressure.
- Calcium carbonate (CaCO3):Calcium carbonate is used as an antacid and a component of cement.
- Sodium bicarbonate (NaHCO3):Sodium bicarbonate is used as a baking soda and an antacid.
Worksheet Analysis
Analyzing data from a worksheet can help us understand the patterns and trends in acids, bases, and salts. By creating a table with columns for acid, base, salt, pH, and reaction type, we can systematically examine the properties and behaviors of these substances.
Data Collection
To populate the table, we can refer to the given acids, bases, and salts worksheet. Each row in the table represents a different acid-base reaction or salt formation process. We extract the relevant information from the worksheet, including the identities of the acid, base, and salt involved, the pH of the solution, and the type of reaction that occurred.
Pattern and Trend Analysis
By examining the completed table, we can identify patterns and trends in the data. For example, we may observe that strong acids tend to produce lower pH values than weak acids, and that the pH of a solution can change significantly depending on the relative strengths of the acid and base involved in the reaction.
Additionally, we may notice that certain types of reactions, such as neutralization reactions, always produce salts with a neutral pH.
These patterns and trends can help us better understand the behavior of acids, bases, and salts, and can inform our predictions about the outcome of future acid-base reactions or salt formation processes.
Real-World Applications
Acids, bases, and salts play vital roles in numerous aspects of our daily lives and various industries. Understanding these concepts is crucial in fields like chemistry, biology, and environmental science.
Everyday Life
- Cleaning products:Acids (e.g., hydrochloric acid) and bases (e.g., sodium hydroxide) are common ingredients in household cleaners for removing stains and dirt.
- Food preservation:Acids (e.g., vinegar) and salts (e.g., sodium benzoate) are used as preservatives in food to prevent spoilage.
- Personal care:Acids (e.g., salicylic acid) and bases (e.g., baking soda) are found in skincare products for treating acne and other skin conditions.
Industry
- Manufacturing:Acids (e.g., sulfuric acid) and bases (e.g., sodium hydroxide) are used in the production of various chemicals, fertilizers, and paper.
- Textile industry:Acids (e.g., acetic acid) and bases (e.g., sodium carbonate) are used in dyeing and treating fabrics.
- Metallurgy:Acids (e.g., hydrochloric acid) and salts (e.g., sodium cyanide) are used in extracting and refining metals.
Science, Acids bases and salts worksheet
- Chemistry:Acids, bases, and salts are fundamental concepts in chemistry, helping us understand chemical reactions and properties of substances.
- Biology:Acids and bases play crucial roles in maintaining pH levels in living organisms, enzymatic reactions, and DNA structure.
- Environmental science:Understanding acids, bases, and salts is essential for monitoring environmental pollution, acid rain, and water treatment.
Interesting Facts
- The strongest known acid is fluoroantimonic acid, which is over a billion times more acidic than sulfuric acid.
- The strongest known base is lithium diisopropylamide, which is over a million times more basic than sodium hydroxide.
- Salt is essential for human health, but excessive consumption can lead to high blood pressure and other health issues.
FAQ Explained
What is the difference between an acid and a base?
Acids are substances that release hydrogen ions (H+) when dissolved in water, while bases release hydroxide ions (OH-) when dissolved in water.
What is pH?
pH is a measure of the acidity or basicity of a solution. It ranges from 0 to 14, with 7 being neutral, values below 7 indicating acidity, and values above 7 indicating basicity.
What is a salt?
A salt is a compound formed by the neutralization reaction between an acid and a base. Salts are typically ionic compounds and can exhibit various properties depending on their composition.