Unravel the fascinating history of the atom project, a captivating tale that has forever transformed our understanding of the world. From the earliest atomic theories to the development of nuclear physics and the Manhattan Project, this journey will illuminate the scientific advancements and ethical dilemmas that have shaped our world.
The quest to understand the fundamental building blocks of matter has been a pursuit that has captivated scientists for centuries. The history of the atom project is a testament to human ingenuity, scientific curiosity, and the transformative power of knowledge.
Early Atomic Theory
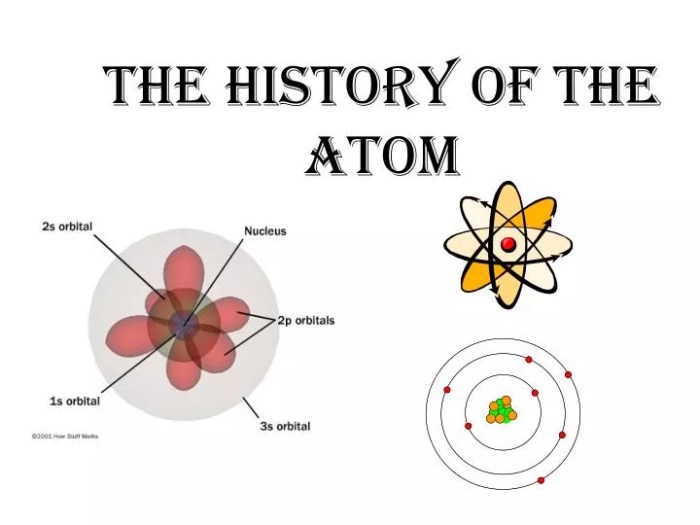
The concept of atoms as the fundamental building blocks of matter has evolved over centuries, with significant contributions from several key figures.
The ancient Greek philosopher Democritus proposed the idea of “atomos,” or indivisible particles, around 400 BC. His student, Leucippus, further developed this concept, suggesting that all matter is composed of these atoms, which differ in size, shape, and arrangement.
John Dalton’s Atomic Theory
In the early 19th century, John Dalton formulated a more comprehensive atomic theory based on his experimental observations. His theory consisted of several key postulates:
- Matter is composed of extremely small, indivisible particles called atoms.
- All atoms of a given element are identical in mass and other properties.
- Atoms of different elements have different masses and properties.
- Atoms cannot be created or destroyed, but they can combine or separate to form compounds.
Dalton’s theory was supported by several experiments and observations, including his work on the behavior of gases and the study of chemical reactions. For example, his experiments on the diffusion of gases showed that gases spread out evenly in a container, indicating that they are composed of tiny, independent particles.
Discovery of the Electron
The discovery of the electron marked a groundbreaking moment in the understanding of atomic structure. In the late 19th century, scientists began to investigate the nature of electricity and its relationship with matter.
One of the key experiments that led to the discovery of the electron was conducted by British physicist J.J. Thomson in 1897. Thomson’s experiment involved passing an electric current through a cathode ray tube, a glass vacuum tube containing two electrodes.
Thomson’s Experiment
- Thomson observed that the cathode ray emitted a stream of particles that traveled towards the positively charged anode.
- He deflected the beam of particles using electric and magnetic fields, demonstrating that the particles had a negative charge.
- By measuring the deflection of the particles, Thomson calculated their mass-to-charge ratio (e/m), which was found to be much smaller than the mass-to-charge ratio of any known atom or ion.
Thomson’s findings provided strong evidence for the existence of a new subatomic particle, which he called the electron. The discovery of the electron revolutionized the understanding of atomic structure, leading to the development of the plum pudding model of the atom, which proposed that electrons were embedded within a positively charged sphere.
Rutherford’s Scattering Experiment
In 1911, Ernest Rutherford conducted a groundbreaking experiment that revolutionized our understanding of the atom. He bombarded a thin sheet of gold foil with a beam of alpha particles (helium nuclei).
The key findings of Rutherford’s experiment were as follows:
- Most alpha particles passed straight through the gold foil without being deflected, indicating that most of the atom is empty space.
- A small number of alpha particles were deflected at large angles, suggesting that there is a small, dense, positively charged nucleus at the center of the atom.
- The number of alpha particles deflected at large angles was very small, indicating that the nucleus is extremely small compared to the size of the atom.
Rutherford’s experiment had profound implications for the structure of the atom. It showed that the atom is not a solid sphere, as previously believed, but rather a mostly empty space with a small, dense nucleus at its center. This model of the atom is known as the Rutherford model and is the basis for our modern understanding of atomic structure.
Bohr’s Model of the Atom
Bohr’s model of the atom, proposed by Niels Bohr in 1913, was a groundbreaking theory that revolutionized our understanding of atomic structure. This model introduced the concept of quantized energy levels and electron transitions, providing a more accurate representation of atomic behavior.
Postulates of Bohr’s Model
The main postulates of Bohr’s model include:*
- *Electrons orbit the nucleus in discrete, circular paths called energy levels.
- *Each energy level has a specific energy associated with it.
- *Electrons can transition between energy levels by absorbing or emitting photons of energy.
- *The angular momentum of an electron in a specific energy level is quantized, meaning it can only take on certain discrete values.
Energy Levels and Electron Transitions
Bohr’s model depicted the atom as a miniature solar system, with electrons orbiting the nucleus like planets around the sun. The energy levels were represented by concentric circles, with the lowest energy level closest to the nucleus.When an electron transitions from a higher energy level to a lower energy level, it emits a photon of energy equal to the difference in energy between the two levels.
Conversely, when an electron absorbs a photon of energy, it transitions to a higher energy level.Bohr’s model successfully explained the observed emission and absorption spectra of hydrogen and other elements. It also provided a theoretical basis for understanding the chemical properties of elements and the formation of chemical bonds.
Quantum Mechanics and the Atom
Quantum mechanics, a fundamental theory in physics, revolutionized the understanding of atomic structure. It introduced groundbreaking principles that challenge classical physics and provide a deeper insight into the behavior of matter at the atomic and subatomic levels.
Wave-Particle Duality
Quantum mechanics postulates that particles, such as electrons, possess both wave-like and particle-like properties. This concept, known as wave-particle duality, challenges the classical view of particles as solely point-like entities.
Uncertainty Principle
The uncertainty principle, a cornerstone of quantum mechanics, states that it is impossible to simultaneously know both the position and momentum of a particle with absolute precision. This principle introduces inherent uncertainty into the behavior of particles at the quantum level.
Quantum States and Energy Levels, History of the atom project
Quantum mechanics describes the energy states of electrons in atoms as discrete and quantized. Electrons can only occupy specific energy levels, and transitions between these levels involve the absorption or emission of energy in the form of photons.
Before diving into the intricacies of the atom project, it’s imperative to address the fundamental question: first and foremost là gì ? This inquiry sets the stage for understanding the motivations and significance of the project, which ultimately led to groundbreaking advancements in our comprehension of the atomic realm.
Electron Configurations
The electron configuration of an atom refers to the arrangement of electrons in its atomic orbitals. The quantum numbers, which describe the properties of electrons, play a crucial role in determining the electron configuration and chemical properties of an atom.
Development of Nuclear Physics
The discovery of radioactivity in the late 19th century revolutionized our understanding of the atom and laid the foundation for nuclear physics. Radioactivity is the spontaneous emission of particles or energy from the nuclei of atoms, indicating that the nucleus is not an indivisible, inert entity.
Marie Curie’s Contributions
Marie Curie, a Polish-French physicist and chemist, played a pivotal role in the early development of nuclear physics. She discovered two new radioactive elements, polonium and radium, and conducted extensive research on the properties of radioactive materials. Her work provided crucial evidence for the existence of subatomic particles within the atom and laid the groundwork for further investigations into the structure of the nucleus.
Ernest Rutherford’s Contributions
Ernest Rutherford, a New Zealand-born British physicist, made significant contributions to nuclear physics. His famous scattering experiment, conducted in 1911, demonstrated that most of an atom’s mass is concentrated in a tiny, dense nucleus at its center. This discovery challenged the prevailing plum pudding model of the atom and paved the way for the development of more accurate atomic models.
The Manhattan Project

The Manhattan Project was a top-secret research and development undertaking during World War II that produced the first atomic bombs. The project was led by the United States with the help of the United Kingdom and Canada.
The goal of the Manhattan Project was to develop an atomic bomb before Nazi Germany could. Scientists involved in the project included J. Robert Oppenheimer, Enrico Fermi, and Leo Szilard.
Scientific and Technological Advancements
The Manhattan Project made significant scientific and technological advancements. These included the development of nuclear reactors, the separation of uranium isotopes, and the design of the atomic bomb.
Ethical and Political Implications
The development of the atomic bomb raised significant ethical and political questions. These included the potential for nuclear war, the threat to civilian populations, and the responsibility of scientists.
Post-War Developments
The end of World War II marked a new era in the history of atomic physics and nuclear technology. The advancements made during the war laid the foundation for numerous groundbreaking applications in various fields.
One of the most significant post-war developments was the advancement of nuclear physics. The Manhattan Project had pushed the boundaries of nuclear science, leading to a deeper understanding of nuclear reactions and the potential for nuclear energy.
Applications of Nuclear Technology
The applications of nuclear technology extended far beyond military purposes. In the field of medicine, the development of nuclear medicine revolutionized diagnostics and treatment methods. Radioisotopes were used for medical imaging techniques, such as X-rays and CT scans, and for cancer treatment through radiation therapy.Nuclear
energy also became a major source of power generation. The construction of nuclear power plants provided a reliable and efficient way to generate electricity, reducing the dependence on fossil fuels and contributing to energy security.Nuclear technology also played a pivotal role in space exploration.
The development of nuclear-powered spacecraft, such as the Voyager probes, enabled scientists to explore the outer planets and interstellar space. These spacecraft utilized nuclear power sources to generate electricity, providing long-term operation capabilities for deep space missions.
FAQ Insights: History Of The Atom Project
What was the significance of Rutherford’s gold foil experiment?
Rutherford’s gold foil experiment provided crucial evidence for the existence of the atomic nucleus. It showed that most of the atom’s mass and positive charge were concentrated in a tiny, dense core, challenging the prevailing “plum pudding” model of the atom.
How did the Manhattan Project contribute to the development of nuclear weapons?
The Manhattan Project was a top-secret research and development effort that produced the first atomic bombs during World War II. It brought together leading scientists and engineers from around the world and resulted in the creation of two types of atomic bombs: a uranium-based bomb and a plutonium-based bomb.
What are the ethical implications of the development of nuclear weapons?
The development of nuclear weapons has raised profound ethical questions about the use of such destructive technology. Concerns include the potential for nuclear war, the long-term health and environmental consequences of nuclear explosions, and the moral responsibility of scientists involved in nuclear weapons research.